Weininger Works™ Open Access Molecule: MS-BLOCK
EV-D68 Summary
Overview of the Isolation of the Structural Correlates of Paralysis and Multiple Sclerosis
MS-BLOCK: The “Weininger MS-Epitope”
The Positions of Myelin P2-like and Toxin-like Domains Relative to the TMEV Capsid Pore
Open Access Molecule Examples
Open Access
Third Party EV-D68-Related References
(WEB PAGE PDF)
EV-D68 Summary
EV-D68 is an enterovirus in the picornavirus family that includes the polio viruses and foot and mouth disease viruses. Tight spatial and temporal clusters of acute flaccid paralysis (AFP) and multiple sclerosis (MS) have been associated with a pathogenic but often initially asymptomatic enterovirus (EV-D68) infection (e.g. Cardiff, UK and Barcelona, Spain); this suggests a common triggering event in each cluster. Asymmetric limb weakness in a child has been found to precede difficulty in walking by two days and subsequent EV-D68-associated death; autopsy samples showed EV-D68 RNA and protein in the anterior horn of the spinal column. Third party patents note common EV-D68 symptoms. Third party reports indicate that EV-D68 infections are underdiagnosed and not rigorously monitored in Europe. This is despite the fact that the tools and methods for detecting any picornavirus, including EV-D68, are readily available and easy to implement. Our (Arthur Weininger and Susan Weininger) limited personal observations are that there is a recent increase in the occurrence of limb flaccidity in the population, consistent with a USCDC Health Alert Network report (CDCHAN-000474).
We are looking for testing partners for an EV-D68/70/71 vaccine and our MS protective compounds, including MS-BLOCK. Properly made MS-BLOCK (matching our patent application coordinates) should be too small to be antigenic but specific enough to not cause antigen broadening. A safe and effective vaccine should be made from an antigen that is unique.
Please contact us with any interest.
Overview of the Isolation of the Structural Correlates of Paralysis and Multiple Sclerosis
Our structural analysis of the picornaviruses (including the enterovirus sub-class containing EV-D68) is given in the following monograph:
https://www.weiningerworks.com/picornavirus_monograph.html (HTML)
(24 MB PDF) (12 MB PDF) (6 MB PDF) (3 MB PDF)
Among other discoveries, we found:
-
unusual toxin-like domains presented on both the outside of the EV-D68 virus protein shell (“capsid”)
and on the outside of the Mahoney poliovirus (PV
1) capsid; and - myelin P2-like domains on the inner shell of Theiler's Murine Encephalomyelitis Virus (TMEV) and EV-D68 capsids.
We concluded that the most likely explanation for:
- paralysis induction by pathogenic enteroviruses is the tight cooperative binding of toxin domains to cellular receptors;
- myelin P2-like domain exposure is the persistent anchoring of multiple toxin substructures on the capsid binding tightly to cellular receptors; and
- MS induction is a matured immune response to a myelin P2-like feature in picornavirus capsids that is presented in an antigenic environment (e.g., exposure near nucleic acid and embedded in a large enough structure).
Myelin P2 injection or infection of mouse with TMEV causes a MS-like condition in the mouse.
EV-D68 is associated with an MS-like condition in humans.
We designed MS-BLOCK to bind to any myelin P2-cross-reacting antibodies induced by EV-D68 myelin P2-like VP1 substructures.
MS-BLOCK are small peptides that present the common feature of myelin, TMEV, and EV-D68 (“Weininger MS-Epitope”).
MS-BLOCK is an Open Access Molecule. We believe that MS-BLOCK compounds have research and clinical purposes. Clinical applications may potentially include detection and treatment of multiple sclerosis (MS) and EV-D68-induced Guillain-Barré Syndrome (GBS).
MS-BLOCK: The “Weininger MS-Epitope”
4WM7, 1TME, and 2WUT are x-ray crystal structures referenced in the Weininger picornavirus monograph.
These crystal structures are shown below.
4WM7 contains structures for EV–D68 capsid proteins: VP1 (white ribbon) and VP2, VP3, VP4 (grey ribbons).
1TME contains structures for TMEV capsid proteins: VP1 (white ribbon) and VP2, VP3, VP4 (grey ribbons).
2WUT contains a structure for myelin P2 protein (white ribbon).
Residues assigned in the x-ray crystal structures as helical have a '•' under the residue.
|
||
4WM7
EV-D68 VP1, VP2, VP3, VP4 capsid proteins
|
1TME
TMEV VP1, VP2, VP3, VP4 capsid proteins
|
2WUT Myelin P2 protein |
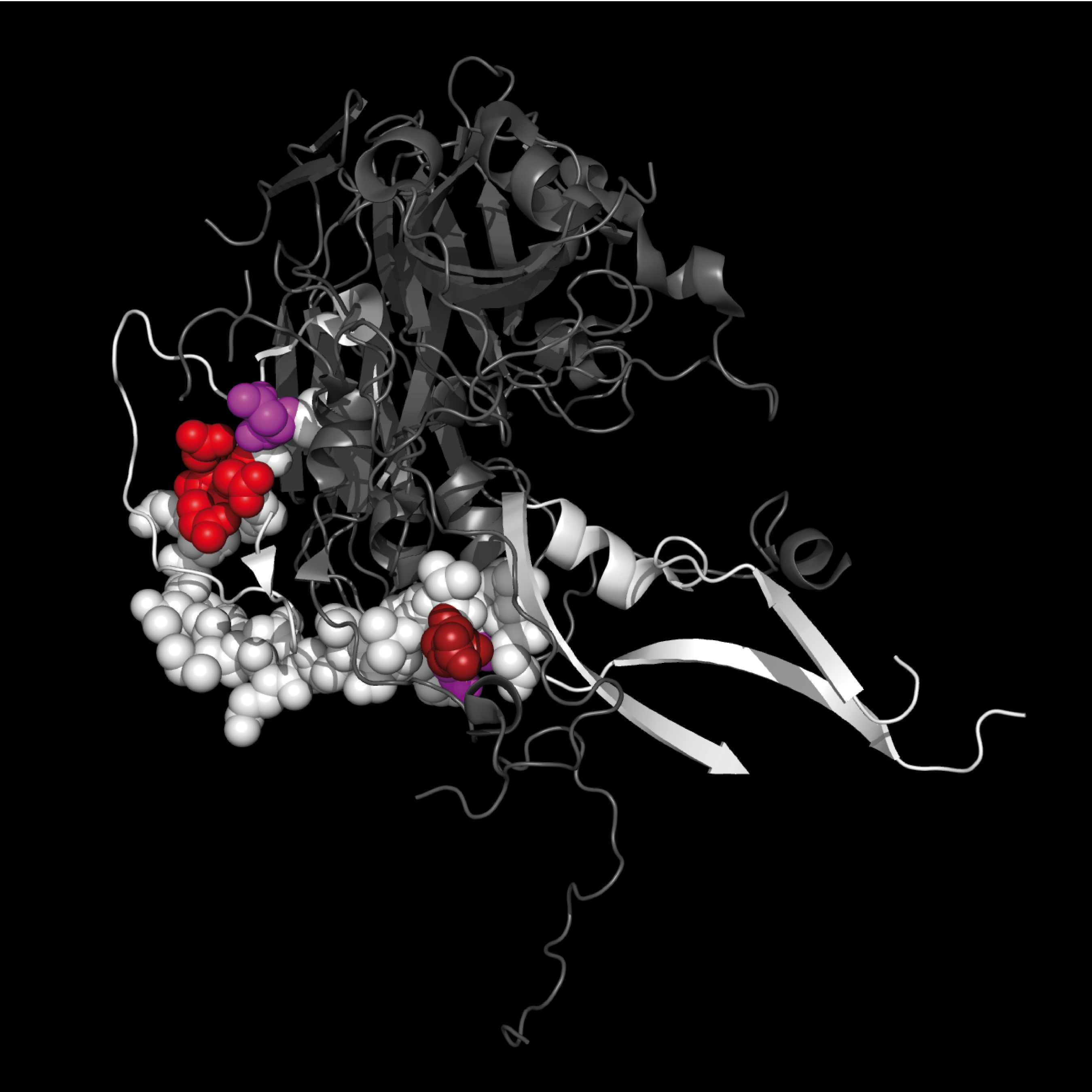 |
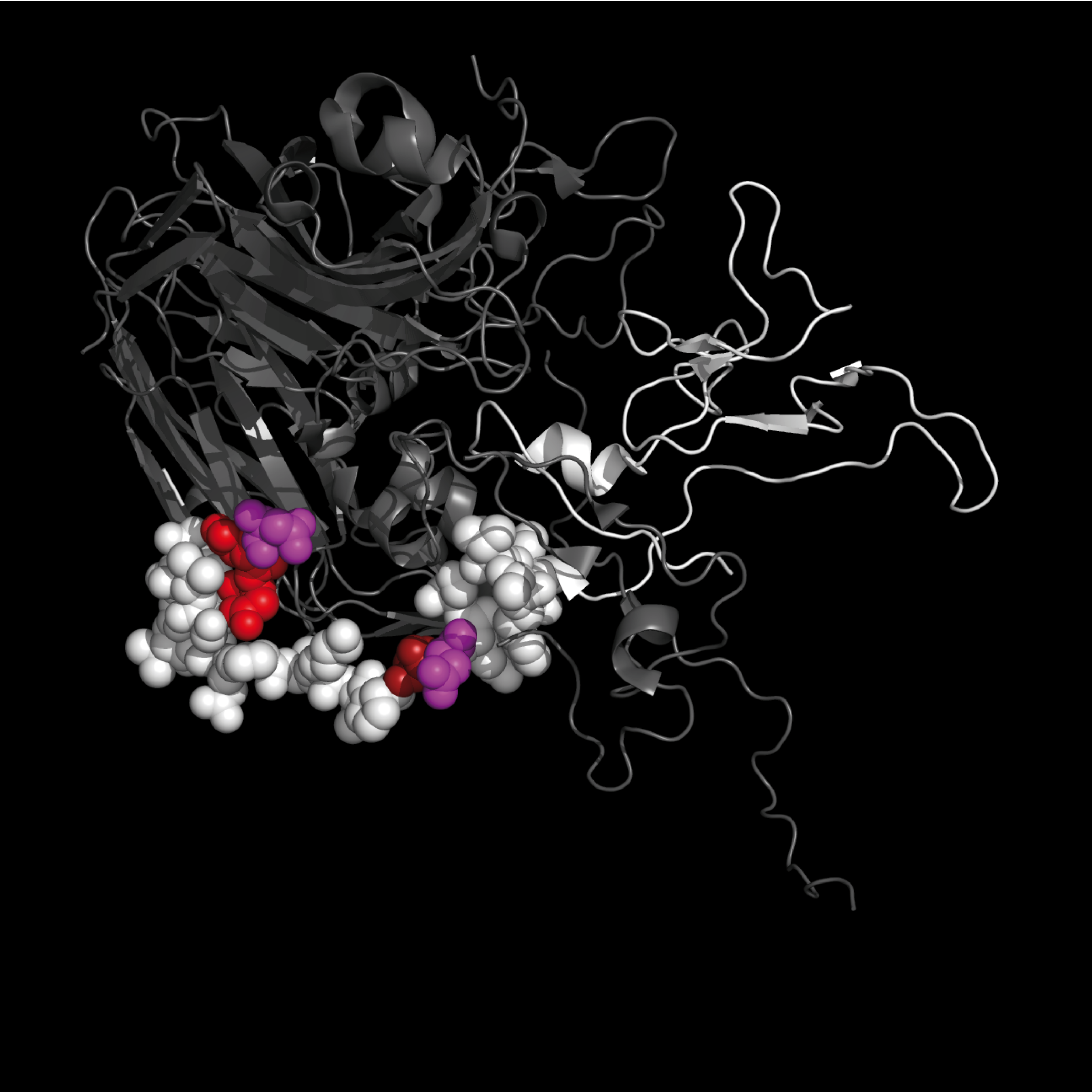 |
 |
Residues S35 – G64 are shown as colored spheres:
SNTEPEEAIQTRTVINQHGVSETLVENFLG
|
Residues S11 – F36 are shown as colored spheres:
SNDDASVDFVAEPVKLPENQTRVAFF
|
Res. S14–K39 are colored spheres:
SSENFDDYMKALGVGLATRKLGNLAK
|
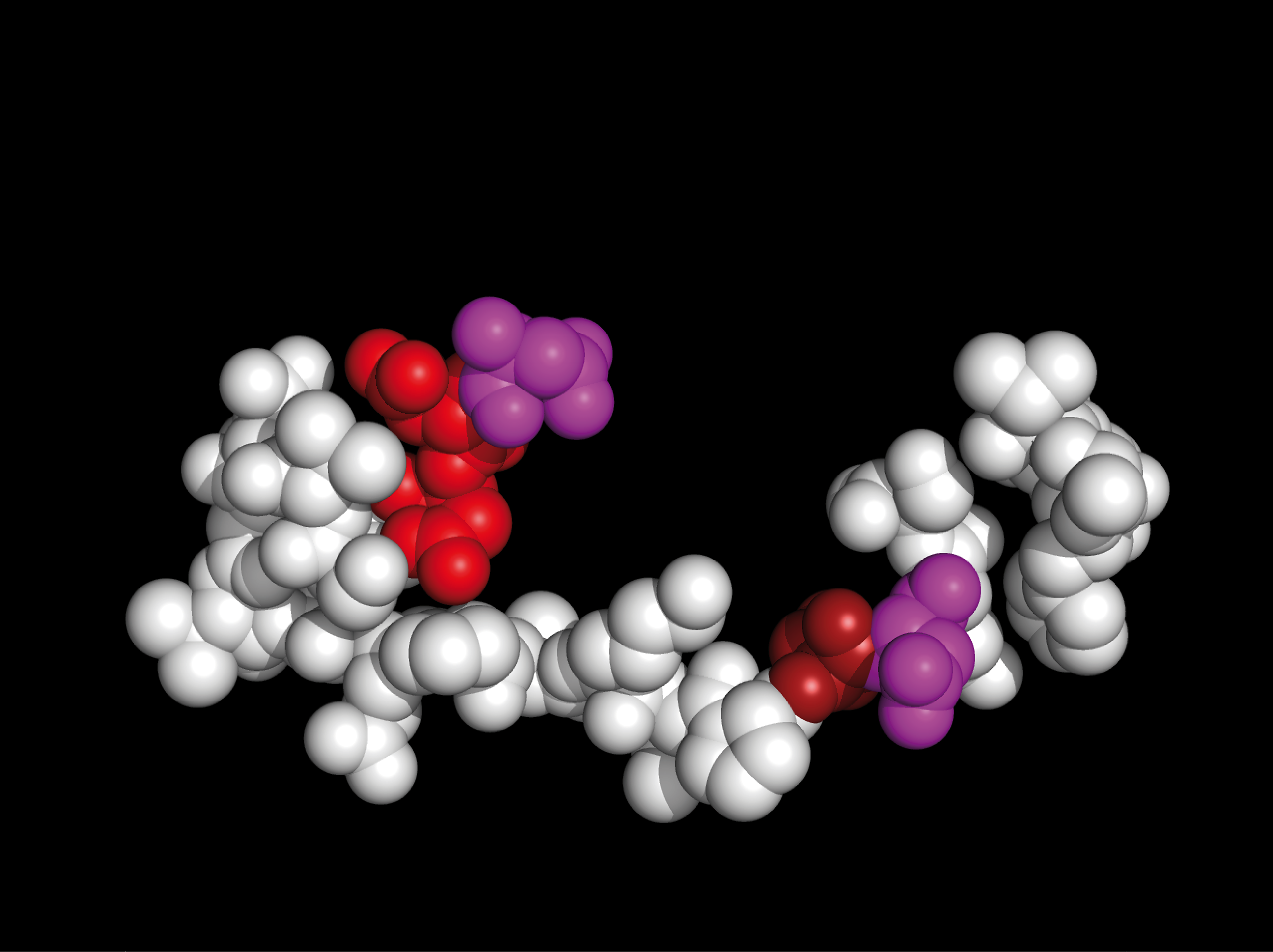
Selected crystallographic substructures of 4WM7 EV-D68 VP1, 1TME TMEV VP1, and 2WUT Myelin P2 are shown below unchanged from their x-ray crystal structures.
Most 4WM7 EV-D68 VP1 and 1TME TMEV VP1 residues in the regions shown below are designated as being in a relatively unstructured loop;
there are few residues assigned as helical secondary structure ('•');
The EV–D68 (4WM7) and TMEV (1TME) substructure residues can be found aligned
with myelin P2 residues in sections M–2, M–3, and M–4 of
Figure 1 of the Weininger picornavirus monograph.
|
||
4WM7
EV-D68 VP1 substructure
|
1TME
TMEV VP1 substructure
|
2WUT Myelin P2 substructure |
 |
 |
 |
EV-D68 VP1 residues S35 – G64are shown as colored spheres:
SNTEPEEAIQTRTVINQHGVSETLVENFLG
|
TMEV VP1 residues S11 – F36are shown as colored spheres:
SNDDASVDFVAEPVKLPENQTRVAFF
|
Myelin P2 protein residues S11 – K39are shown as colored spheres:
SSENFDDYMKALGVGLATRKLGNLAK
|
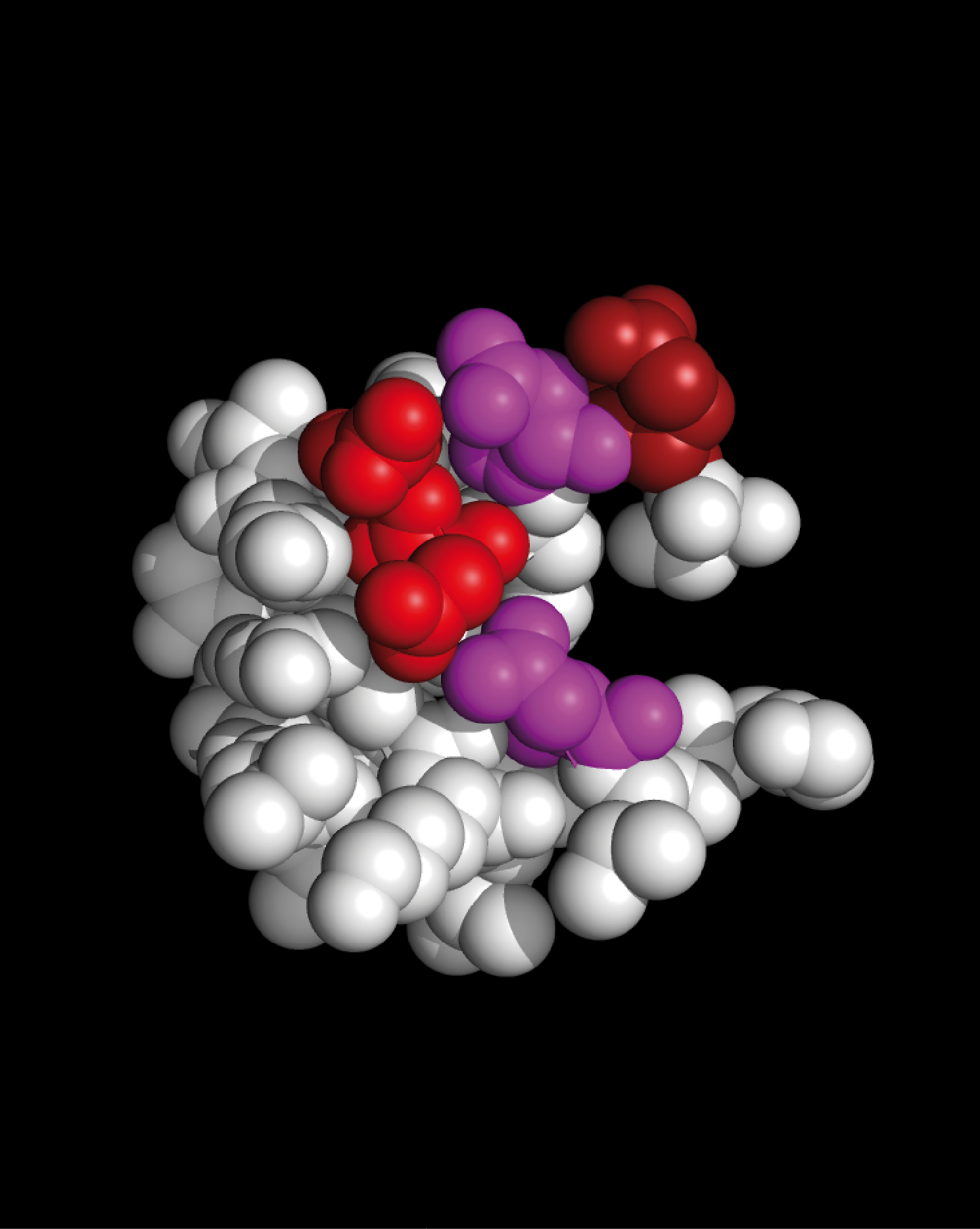
The Weininger generated structures of the 4WM7 VP1 residues and 1TME VP1 residues shown below contain the same residues
shown above as spheres,
but the residues are formed into a helix-loop-helix structure.
The Weininger generated VP1 substructures of 4WM7 and 1TME present a similar set of charged residues to myelin P2 protein.
We call this common structural feature the “Weininger MS–Epitope”
and it forms the basis for the small molecule protein set MS–BLOCK.
Residues that have been formed into helices in the Weininger generated structures have a '♦' under the residue.
|
||
|
Weininger MS-Epitope
Structure Fragment
Selected EV-D68 VP 1 Residues
|
Weininger MS-Epitope
Structure Fragment
Selected TMEV-VP 1 Residues
|
Weininger MS-BLOCK
Selected Myelin-P2 Residues |
 |
 |
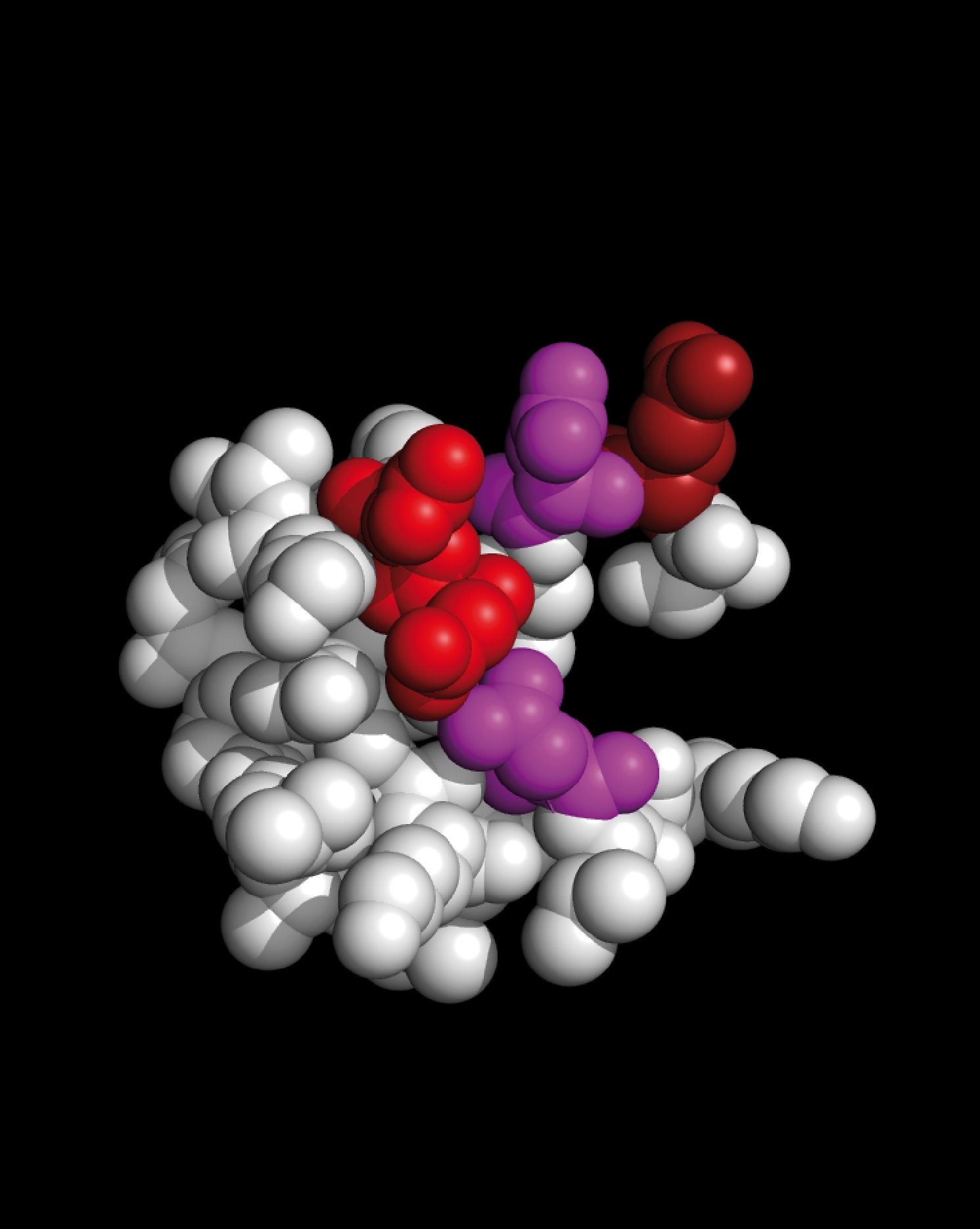 |
EV-D68 VP1 residues S35 – G64are shown as colored spheres:
SNTEPEEAIQTRTVINQHGVSETLVENFLG
|
TMEV VP1 residues S11 – F36are shown as colored spheres:
SNDDASVDFVAEPVKLPENQTRVAFF
|
Myelin P2 protein residues S14 – K39are shown as colored spheres:
SSENFDDYMKALGVGLATRKLGNLAK
|
The Positions of Myelin P2-like and Toxin-like Domains Relative to the TMEV Capsid Pore
1TME and 1TMF
are x-ray crystal structures referenced in the Weininger picornavirus monograph.
1TME and 1TMF are both structures of Theiler's Murine Encephalomyelitis Virus (TMEV), a picornavirus known to cause an MS-like condition in mice.
The structure of the picornavirus capsid is an icosahedron.
1TME and 1TMF each contain structures for a set of capsid proteins (VP1, VP2, VP3, and VP4).
Sixty of these sets (3 times per side for 20 capsid tiling pieces) comprise the capsid.
The crystal structures shown below display a subset of a modeled capsid: a pentamer of tiling pieces.
Although 1TME and 1TMF have nearly identical sequences
they present related but different configurations
of the VP1, VP2, VP3 and VP4 capsid proteins relative to one another.
1TME (TMEV) has a similar presentation to other picornavirus crystal structures, including 4WM7 (EV-D68) and 1HXS (Mahoney polio virus);
we call this structural presentation CASOG-1 (Common Atomic Structural Occupancy Group 1).
1TMF (TMEV) has a similar presentation to other picornavirus crystal structures, including 1BBT (FMDV);
we call this structural presentation CASOG-2 (Common Atomic Structural Occupancy Group 2).
CASOG-1 proteins in a capsid form open pores. CASOG-2 proteins in a capsid form closed pores.
The figures below show the positions of pores, myelin P2-like sequences and the positions of residues
in the I1-1, I1-2, I1-3, I1-4, I1-5, and A1 Sections of Figure 1
of the Weininger picornavirus monograph.
1TME
– TMEV capsid inside (CASOG 1 - OPEN PORE)
|
1TME
– TMEV capsid outside (CASOG 1 - OPEN PORE)
|
 |
 |
1TMF
– TMEV capsid inside (CASOG 2 - CLOSED PORE)
|
1TMF
– TMEV capsid outside (CASOG 2 - CLOSED PORE)
|
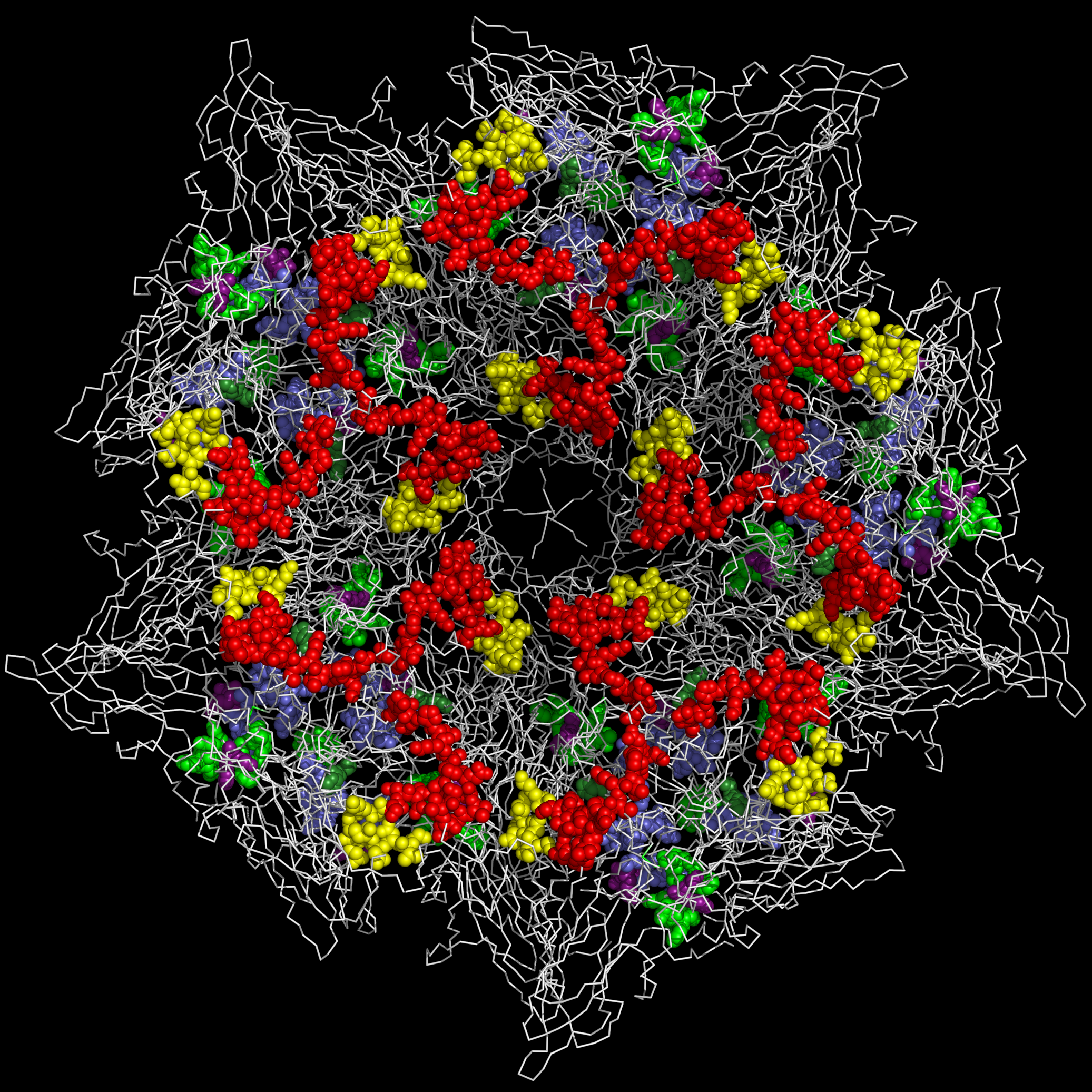 |
 |
It can be seen from the figures above that:
- the myelin P2-like sequences in TMEV (and by extension EV-D68 and Mahoney polio virus) are presented at the rims of their pores and can be extruded to the exterior of the capsid along with the RNA when the capsid is bound to cellular receptors and the pore is opened; and
-
the highly variable residues in the Figure
1sectionsI1-1andI1-2are presented on the outside of the capsid at the center of the capsid tiling piece.
In EV-D68 and Mahoney polio virus, the Figure 1 Sections I1-1, I1-2, and I1-4 present residue loops found commonly in toxins.
FIGURE 1 SECTIONS RESIDUES
M1 ................. VP1 N-terminal residues
M2 – M4 ............ VP1 Myelin P2-like residues
I1-1 ............... VP1 highly variable I1-1 Insert residues
I1-2 ............... VP1 highly variable I1-2 Insert residues containing the position
(bounded by KTK in TMEV) of the insertion of toxin-like residues
in EV-D68 and Mahoney Polio Virus
I1-3 ............... VP1 highly variable I1-3 Insert residues
A1 ................. VP1 A1 GW residues mark the position of the toxin-like residues
in EV-D68 and Mahoney Polio Virus
I1-4 ............... VP1 highly variable I1-4 Insert residues
I2-5 ............... TMEV VP1 residues PT are found in Figure 1 to bracket (but are not
included in) the highly variable I2-5 as the I2-5 Insert residues
found in other picornaviruses are missing entirely in TMEV.
TMEV VP1 residues PT are shown to indicate the position of
IL-5 Insert residues in other picornaviruses including EV-D68.
I123-1 I123-2 I13-1
| | |
<---M1---><---M2---->*<M3->*<-M4------><------M5------>***<-I1-1-><--B2--><B3>
1TME:GSDNAEKGKVSNDDASVDFVAEPVKLPENQTRVAFFYDRAVPIGMLRPGQNIESTFVYQENDLRLNCLLLTPLPSFC
1TMF:GVDNAEKGKVSNDDASVDFVAEPVKLPENQTRVAFFYDRAVPIGMLRPGQNMETTFNYQENDYRLNCLLLTPLPSFC
I1-3 I123-6 M11 I123-7
I123-3 | I123-4 M8 | M9 |I13-2|
| | | | | | | | |
<---I1-2---><-B3-><***><*>**<------B5------><-----M6------><M7>*<**>*<*><*>**
1TME:PDSTSGPVKTKAPVQWRWVRSGG••TTNFPLMTKQDYAFLCFSPFTYYKCDLEVTVSALGTDTVASVLRWAPTGAPAD
1TMF:PDSSSGPQKTKAPVQWRWVRSGGVNGANFPLMTKQDYAFLCFSPFTFYKCDLEVTVSALGTDTVASVLRWAPTGAPAD
<--------M12--------><-----------------A1----------><---I1-4----><--A2-->
1TME:VTDQLIGYTPSLGETRNPHMWLVGAGNTQISFVVPYNSPLSVLPAAWFNGWSDFGNTKDFGVAPNADFGRLWI
1TMF:VTDQLIGYTPSLGETRNPHMWLVGAGNSQVSFVVPYNSPLSVLPAAWFNGWSDFGNTKDFGVAPNADFGRLWI
I2-6
|
<M13-><-----M14------><--I2-5---><-M15->**<-------M16------->
1TME:QGNTSASVRIRYKKMKVFCPRP•••••••••••TLFFPW••PVSTRSKINADNPVPILELE
1TMF:QGNTSASVRIRYKKMKVFCPRP•••••••••••TLFFPWPTP•TT•TKINADNPVPILELE
MS-BLOCK Open Access Molecule Examples
The following cyclic peptides are example formulations of MS-BLOCK. These cyclic peptides are expected to bind antibodies to any of the myelin P2-like helices or CRABP-like helices found on EV-D68 and TMEV. The antibodies that bind to MS-BLOCK are expected to cross-react with myelin P2 helices and CRABP helices.
The following images show the structures and sequences of two MS-BLOCK cyclized peptides derived, separately, from myelin P2 protein and CRABP.
 |
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CYCLIZED PEPTIDE DERIVED FROM MYELIN P2 MCLVSSENFDDYMKALGVGLATRKLGNLAKPCGV |
CYCLIZED PEPTIDE DERIVED FROM CRABP MCIIRSENFEELLKVLGVNVMLRKIAVAAASKPCGV |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Residues presented as spheres are colored as follows: ARG LYS GLU ASP ASN CYS GLY PRO |
Residues presented as spheres are colored as follows: ARG LYS GLU ASN CYS GLY PRO |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Color coding and sequences of residues shown above:
|
Color coding and sequences of residues shown above:
|
MS-BLOCK Open Access Molecule
Arthur Weininger and Susan Weininger filed and then abandoned Israel Patent Application #235702 (PDF) for MS-BLOCK. Abandonment ensured that the claims of Israel Patent Application #235702 would not act as a bar to third party practice. There are no actual or potential foreign patent applications corresponding to Israeli Patent Application #235702 currently pending in any territory.
Open Access Molecules (Definition and Disclaimers)
“Open Access Molecules” are specific molecules upon which Arthur Weininger and Susan Weininger make no proprietary claim.
Open Access Molecules listed by Arthur Weininger and Susan Weininger are provided “as is” and without warranty of any kind.
No oral or written information or advice given by Arthur Weininger and/or Susan Weininger shall create any warranty with regards to Open Access Molecules.
Open Access Molecules may not be: useful, safe, or non-infringing of third party rights.
Arthur Weininger and Susan Weininger do not agree to any indemnification for anything related to Open Access Molecules including, but not limited to,
any use of Open Access Molecules, any legal actions directly or indirectly associated with Open Access Molecules, or any losses or damages directly or indirectly associated with Open Access Molecules.
In no event shall the total liability by Arthur Weininger and Susan Weininger for any and all damages related to Open Access Molecules exceed zero.
Any use of Open Access Molecules is at your sole and entire risk.
Third Party EV-D68-Related References
The following study reported that EV-D68 RNA and protein was detected in anterior horn motor neurons and their axons in the autopsy tissue of a 5 year old boy.
Two days before the boy began to have difficulty walking, he exhibited asymmetric arm weakness.
Guillain-Barré Syndrome (GBS) has been found to be associated with EV-D68 infection:
The following study of EV-D68 hospitalized children states “The potential neurotropism indicates that enterovirus surveillance should be mandatory.”:
The following study states “Enteroviruses (EV) can cause severe neurological and respiratory infections,
and occasionally lead to devastating outbreaks as previously demonstrated with EV-A71 and EV-D68 in Europe.
However, these infections are still often underdiagnosed and EV typing data is not currently collected at European level.”:
The following USCDC Health Alert Network report states “In August 2022,
CDC was notifed by healthcare providers and hospitals in several regions of the United States
of increases in severe respiratory illness in children who also tested positive for RV/EV.
Consistent with this, an increase in respiratory specimens positive for RV and/or EV
was noted in the National Respiratory and Enteric Virus Surveillance System (NREVSS).
In addition, CDC monitors EV-D68 detections across the New Vaccine Surveillance Network (NVSN),
a platform of seven U.S. medical centers that perform active,
prospective surveillance for pediatric acute respiratory illness.
Between April – August 2022, EV-D68 was detected in some children and adolescents with ARI across all seven sites.
The number of detections in July – August 2022 was greater than in the same period of the previous three years (2019, 2020, and 2021).
As of August 30, 2022, CDC had not received increased reports of acute accid myelitis (AFM) cases with onset in 2022.
However, increases in EV-D68 respiratory illnesses have typically preceded cases of AFM,
indicating that increased vigilance for AFM in the coming weeks will be essential.
”:
Multiple protocols for testing picornaviruses (e.g. polio) have been evaluated and are available:
The United State Patents #9,872,900,
#10,022,435, and
#10,709,779
(all assigned to ModernaTX, Inc. Cambridge MA) all state:
1 and Human enterovirus 68 Enterovirus 71 (EV-71)
is one of the major causative agents for hand, foot and mouth disease (HFMD),
and is sometimes associated with severe central nervous system diseases.
The Enterovirus 71 (EV71) infection may be asymptomatic.”
1962, it has been been on a worldwide upswing in the last few years.
It may be involved in cases of a recent outbreak of polio-like disease in California.”