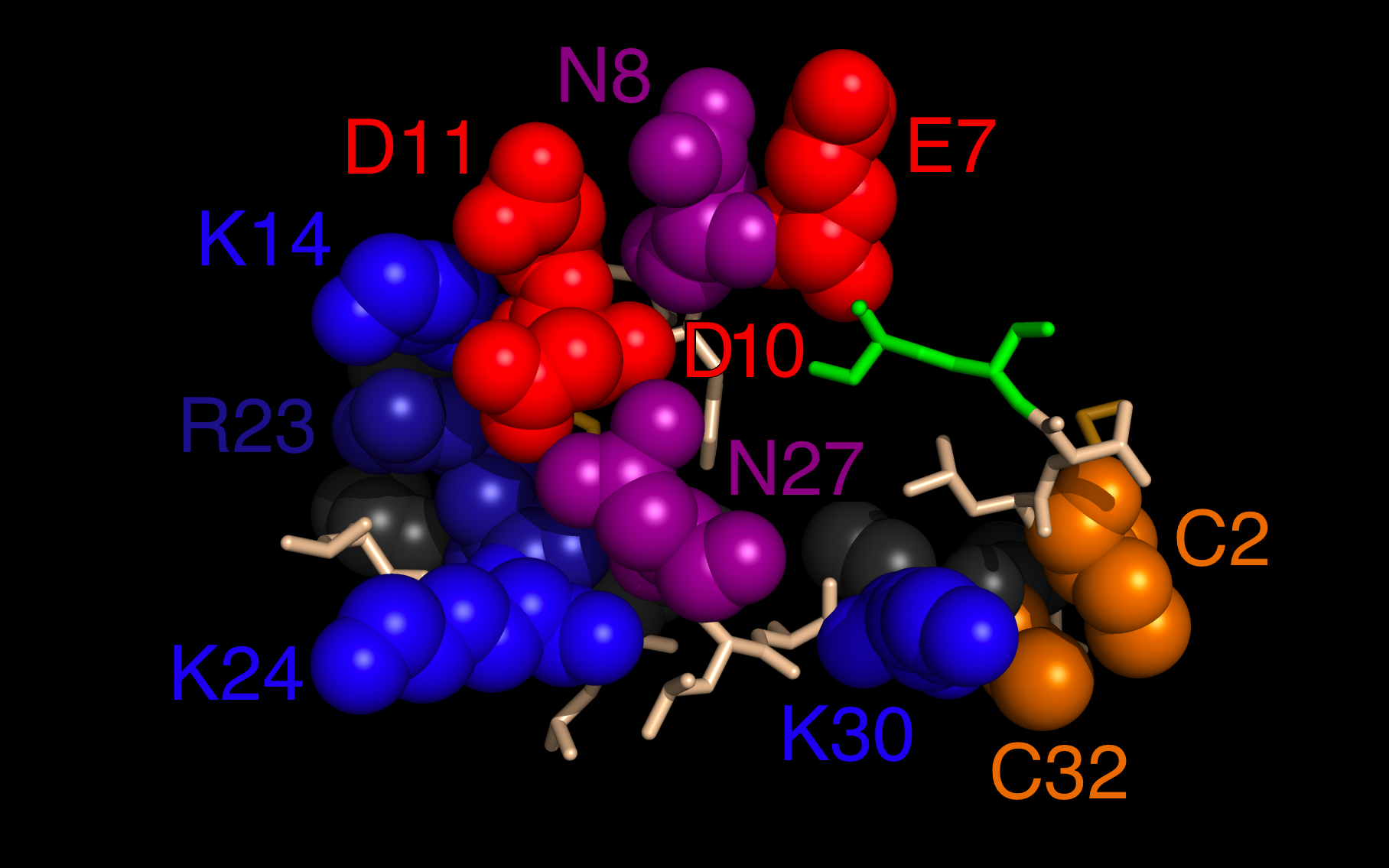
MS-BLOCK / EV-D68 |

FLU-LOCK |
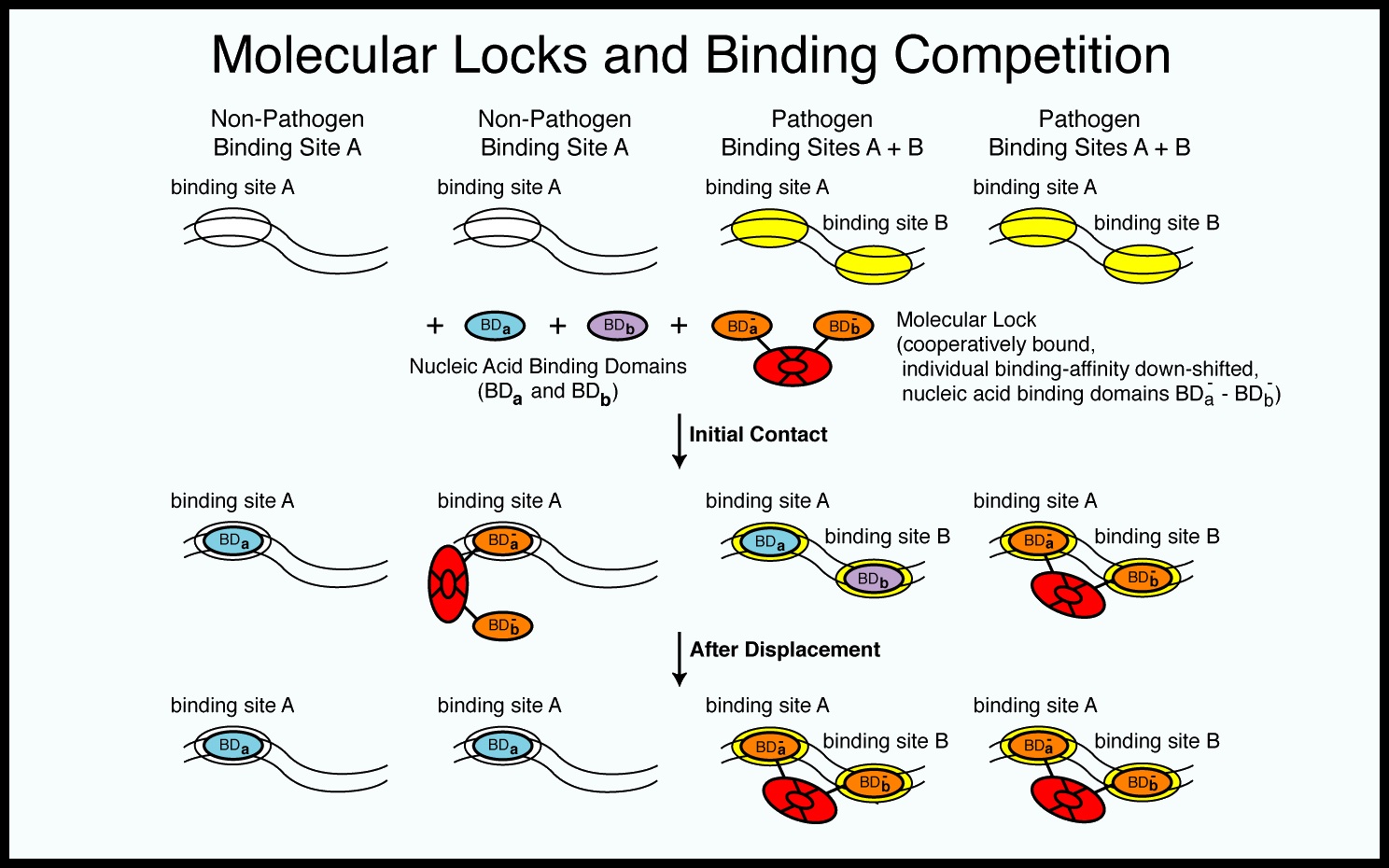
Molecular Locks/HIV-LOCK |
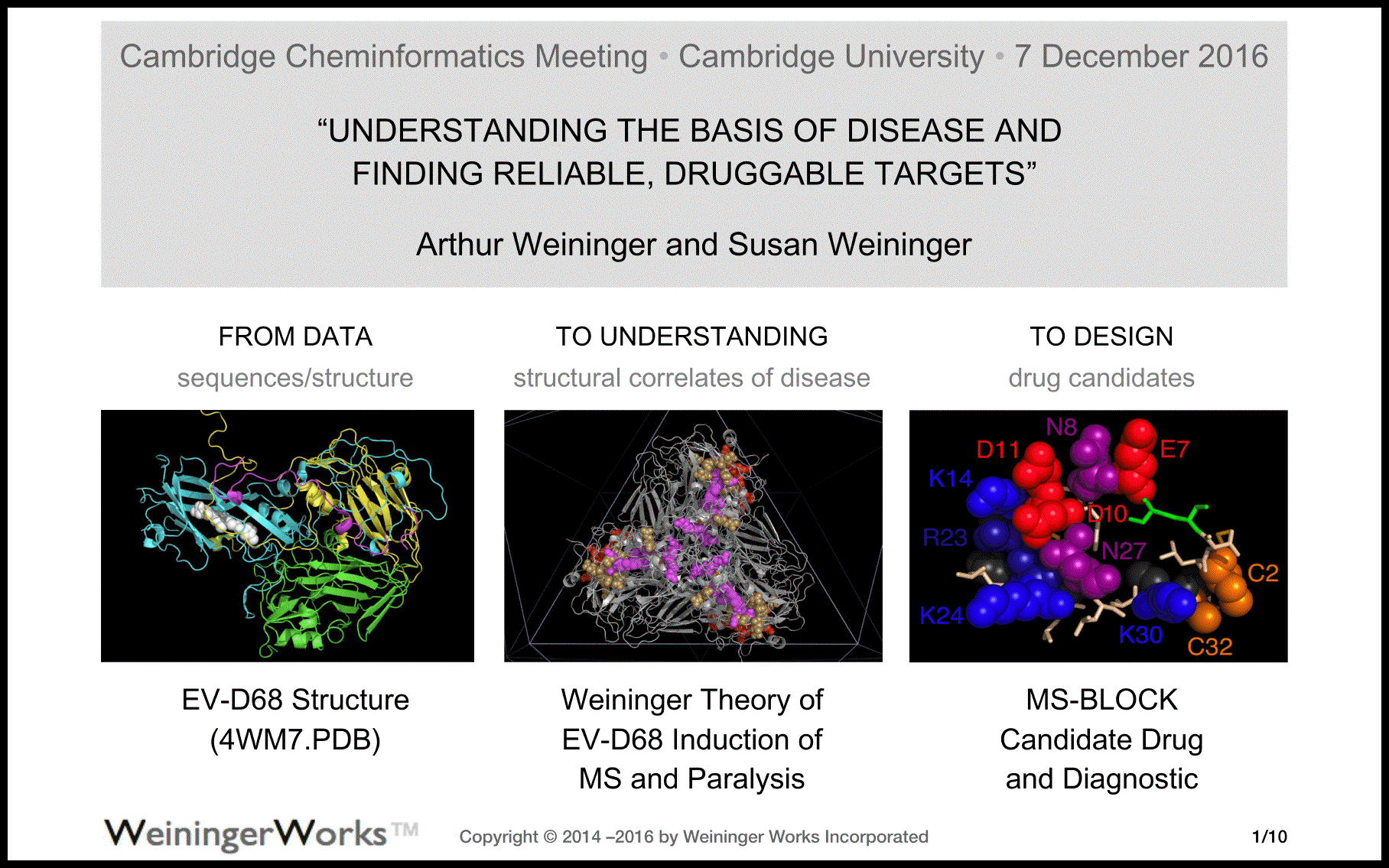
Lectures & Patents |

Analytics Tests |
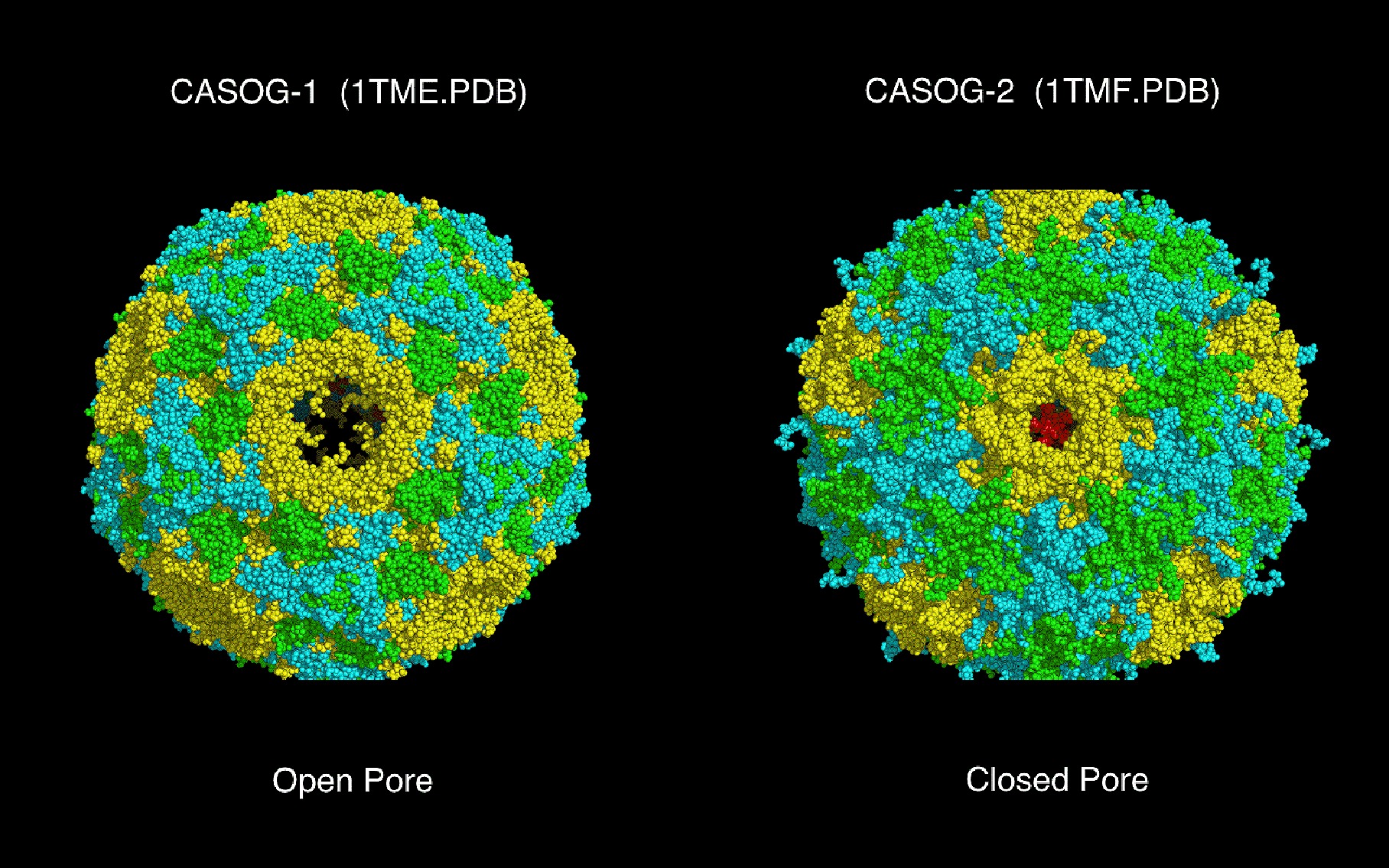
Software |
| W einingerW orks | MS-BLOCK EV-D68 |
FLU-LOCK | Molecular Locks/HIV | Lectures & Patents | Analytics Tests | Software | Contact |